Most modern active pharmaceutical ingredients (APIs) are complex molecules with often more than one stereocenter. A major advancement on the synthesis of these molecules would be to develop a robust process to obtain two stereocenters in a single step.
Our focus is on non-natural amino acids, building blocks which have expanded the range of peptide-based drugs. Non-natural amino acids with two stereocenters are extremely challenging to produce, with low supply available. However, there is demand for these compounds, illustrated by the development and commercialization of peptide-based drugs containing these building blocks, such as Paxlovid ©, developed by Pfizer for the treatment of COVID-19, and Retosiban© developed by GSK as an oxytocin antagonist to prevent preterm labour.
Today, our partner ChiralVision produces Isoleucine stereoisomers using a lengthy chemo-enzymatic route, and have established themselves in the market. However, this 8-step route is challenging to scale-up, and therefore due to limited capabilities it is on back order because of the high demand of clients.
Our approach: by investigating and employing new enzymes as bio-catalysts, we will generate two stereo-centers in a single synthetic step. Simultaneously, we will implement enzyme immobilization based on renewable carriers (ChiralVision) and cross-linked enzyme nano-aggregates in the nano-cavity of bowl-shaped polymer vesicles (c-CLEnA). Established co-factor recycling methodologies developed for the ONE-FLOW project will also be used. Combined with the innovative rotating bed reactors developed by SpinChem, these technologies will allow for efficient bio-catalyst separation and reuse.
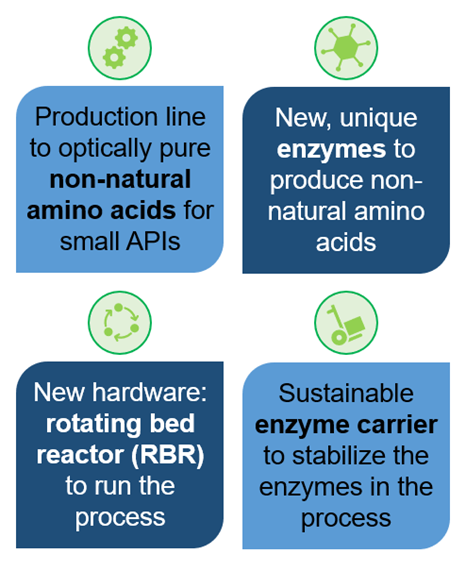
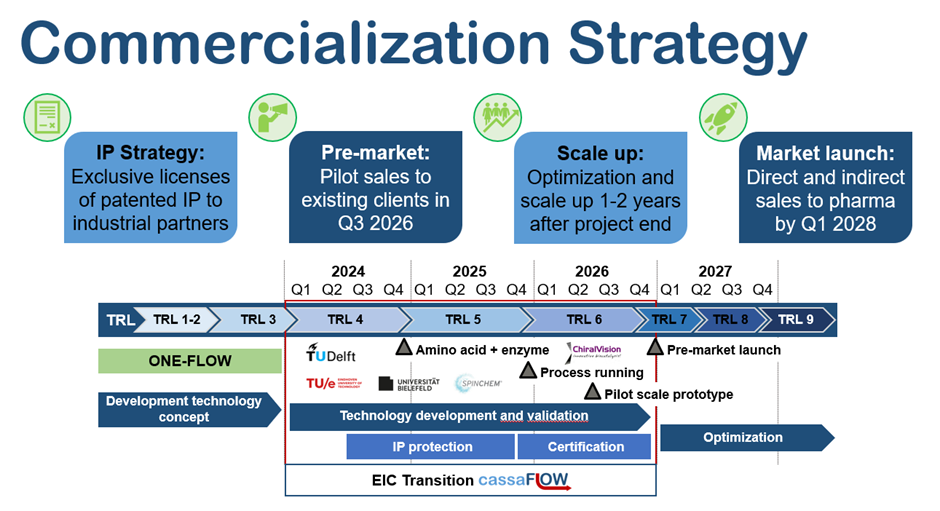
We aim to develop our products to TRL 6 by the end of the EU-funded project (Q4 2026).
Interested in knowing more about our industrial partners? Visit their websites for more information:
